Abstract
* Contributed equally to this work
Background
Checkpoint proteins regulate the immune system and their down-regulation of effector mechanisms is exploited by malignant cells to evade antitumor response. Membrane bound programmed death 1 protein (PD-1) and its ligand (PD-L1) are checkpoint proteins whose over-expression has been reported as an adverse prognostic factor in lymphoid malignancies. Monoclonal antibodies targeting these checkpoint proteins are therefore a rational therapy trying to re-establish an effective antitumor immunity.
Soluble forms of PD-1 and PD-L1 (sPD-1 and sPD-L1) exist, but their biological and clinical significance is yet to be clarified. High pre-therapeutic levels of sPD-L1 have been reported to impact overall survival in diffuse large B cell lymphomas (DLBCL). However, the role and significance of soluble levels of the receptor protein sPD-1 are still largely unknown.
Aims
The aims of the present study were to measure pre-therapeutic sPD-1 levels in selected lymphoid malignancies and, for de novo DLBCL, investigate the potential correlation with outcome-related parameters.
Methods
We established and validated a Time Resolved Immunoflourometric assay (TRIFMA) to determine levels of sPD-1. In total, 106 archival serum samples were analysed in duplicate. This single-institution study population consisted of patients with chronic lymphocytic leukemia (CLL; n=42), DLBCL (n=30), Hodgkin lymphoma (HL; n=12), and healthy controls (n=22).
Information on clinical parameters was obtained from medical records. sPD-1 levels were compared between patient subgroups and controls using a non-parametric test (Mann-Whitney). Correlation with clinical parameters were analysed using a linear regression model and Spearman's rank correlation. P-values below 0.05 were considered statistically significant.
Results
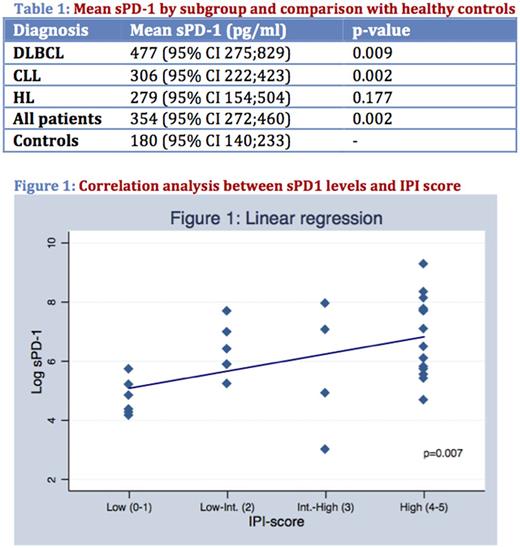
In general, sPD-1 levels were higher in patients, taken as one group, compared to controls (p=0.002, see table 1). When analysed according to individual diagnostic subgroups, patients with DLBCL and CLL still had, despite of their limited cohort size, significantly elevated sPD-1 levels compared to controls (p=0.009 and p=0.002, respectively), while patients with HL did not. Although not significantly different, DLBCL patients showed consistently higher mean values of sPD-1 compared to CLL and HL (see table 1).
A separate analysis of patients with DLBCL revealed a positive correlation between pre-therapeutic sPD-1 and IPI-score (β1=0.579 (95% CI 0.169;0.990), p=0.007)(see figure 1). The correlation was confirmed with Spearman's test of no association (p=0.0043). More specifically, higher sPD-1 levels correlated with higher IPI score and with the presence of known adverse prognostic factors such as age >60 years, disseminated clinical stage, elevated p-LDH, ECOG performance score ≥2, presence of >1 extranodal site.
DLBCL patents were also analysed according to whether their tumor cell population was of GCB (n=14) or non-GCB (n=16) origin (assessed by the immunohistochemistry-based Hans algorithm). Non-GCB cases (mean 671 pg/ml (95% CI 268; 1680)) had higher sPD-1 levels than GCB cases (mean 323 pg/ml (95% CI 174; 560)), however not statistically significant (p=0.1575).
Conclusion
Pre-therapeutic serum levels of sPD-1 were found to be higher in selected lymphoid malignancies (DLBCL, CLL) than in healthy controls. Highest mean values were detected in DLBCL patients, where higher sPD-1 levels also significantly correlated to the presence of classical clinical prognosticators for adverse outcome and, possibly, also to a non-GCB phenotype. Correlation analyses between pre-therapeutic levels of sPD-1, sPD-L1 and clinico-pathological features as well as clinical behaviour and outcome are ongoing.
Clausen: Takeda: Research Funding. d'Amore: Nordic Nanovector: Other: Advisory Board; Les Laboratoires Servier: Honoraria, Other: Advisory Board; Takeda Pharma: Honoraria, Other: Advisory Board, Research Funding; Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal